Researchers develop fastest-of-its-kind carbon storage technology
Technology tamfitronics
Researchers from theUniversity of Texas at Austinhave devised a faster method for storing captured carbon from the atmosphere. This method eliminates the need for harmful chemical accelerants usually required by current storage methods.
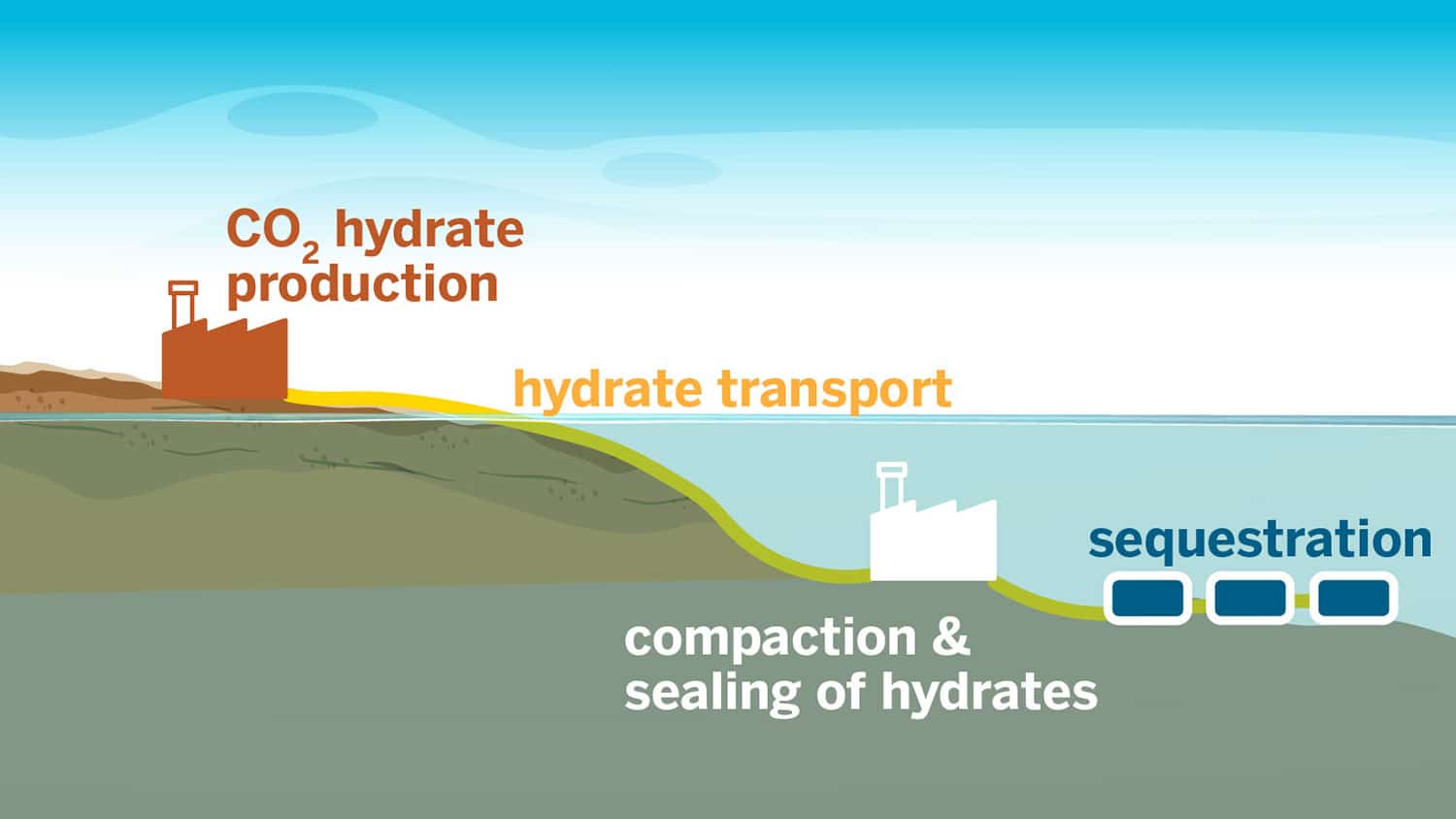
The team developed a technique for ultrafast formation of carbon dioxide hydrates. These distinctive ice-like substances can sequester carbon dioxide in the ocean, effectively preventing its release into the atmosphere.
“We’re staring at a huge challenge – finding a way to safely remove gigatons of carbon from our atmosphere – and hydrates offer a universal solution for carbon storage. For them to be a major piece of the carbon storage pie, we need the technology to grow them rapidly and at scale,” said Vaibhav Bahadur, a professor in the Walker Department of Mechanical Engineering who led the research. “We’ve shown that we can quickly grow hydrates without using any chemicals that offset the environmental benefits of carbon capture.”
Carbon dioxide stands as the prevalent greenhouse gas and plays a significant role in driving climate change. The process of carbon capture and sequestration works to remove carbon from the atmosphere and maintain its storage indefinitely. It is considered an essential element in the effort to reduce carbon emissions and mitigate the impact of climate change.

Currently, the predominant method for storing carbon involves the injection of carbon dioxide into underground reservoirs. This approach offers the dual advantages of carbon containment and the stimulation of oil production.
However, this technique faces significant challenges, such as the escape and movement of carbon dioxide, contamination of groundwater, and the potential for seismic activity linked to injection. Additionally, many regions around the world do not have suitable geological characteristics for reservoir injection.
According to Bahadur, hydrates are viewed as a secondary option for large-scale carbon storage, but they could become the primary option if key challenges are addressed. Up to this point, the process of creating these hydrates that trap carbon has been sluggish and requires a lot of energy, which has hindered its adoption as a widespread method of carbon storage.
In a recent study, researchers have achieved a sixfold increase in the rate of hydrate formation compared to previous techniques. The rapid speed, in combination with the chemical-free process, simplifies the utilization of these hydrates for large-scale carbon storage.

Magnesium plays a crucial role in this research, serving as a catalyst that eliminates the requirement for chemical promoters. This is supported by the high flow rate of CO2 bubbling in a specific reactor setup. The technology is compatible with seawater, making it easier to deploy as it does not depend on complex desalination processes to produce fresh water.
“Hydrates are attractive carbon storage options since the seabed offers stable thermodynamic conditions, which protects them from decomposing,” Brave said. “We are essentially making carbon storage available to every country on the planet that has a coastline; this makes storage more accessible and feasible on a global scale and brings us closer to achieving a sustainable future.”
This breakthrough has implications that go beyond just carbon sequestration. The rapid formation of hydrates could be used in desalination, gas separation, and gas storage, providing a flexible solution for different industries. The University of Texas researchers have applied for two patents related to the technology, and they are exploring the possibility of launching a startup to bring it to market.
Journal reference:
- Awan Bhati, Mark Hamalian, Palash V. Acharya, and Vaibhav Bahadur. Ultrafast Formation of Carbon Dioxide Hydrate Foam for Carbon Sequestration. ACS Sustainable Chemistry & Engineering2024; DOI: 10.1021/acssuschemeng.4c03809
Discover more from Tamfis Nigeria Lmited
Subscribe to get the latest posts sent to your email.



 Hot Deals
Hot Deals Shopfinish
Shopfinish Shop
Shop Appliances
Appliances Babies & Kids
Babies & Kids Best Selling
Best Selling Books
Books Consumer Electronics
Consumer Electronics Furniture
Furniture Home & Kitchen
Home & Kitchen Jewelry
Jewelry Luxury & Beauty
Luxury & Beauty Shoes
Shoes Training & Certifications
Training & Certifications Wears & Clothings
Wears & Clothings
















