Amyloid is everywhere, but new treatments could stop the toxic build up
Technology tamfitronics Amyloid deposits are present in the brains of cognitively impaired individuals, but also accumulate throughout the body. Some biotechs aim to keep these misfolded proteins in check to treat a range of diseases, including heart failure, ALS and Alzheimer’s disease.
By late November, the US Food and Drug Administration (FDA) will decide whether to approve a highly anticipated new drug for treating a form of amyloid buildup that leads to heart failure. BridgeBio has developed acoramidis for treating transthyretin amyloidosis with cardiomyopathy (ATTR-CM), an important but often undiagnosed cause of heart failure that has become a significant focus for drug developers. Alnylam Pharmaceuticals is targeting this protein misfolding condition with another treatment option, Amvuttra (vutrisiran). The same drug is already approved for treating hereditary forms of ATTR with polyneuropathy, a progressive and fatal disease characterized by damage to the peripheral nerves. There is more to come, too, as a broad pipeline of amyloid-targeting therapies is in late clinical development (Table 1).
Full size table

The amyloid-β molecule folds and stacks to form insoluble fibrils that cause pathologies when deposited in tissues. Credit: VD Image Lab/Alamy Stock Photo
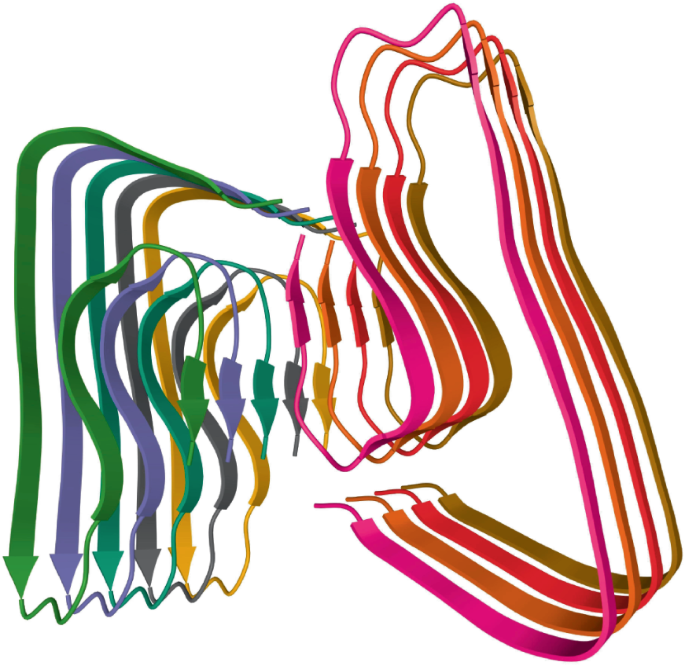
In all, some 36 human ‘amyloidogenic’ proteins have been identified. These proteins are all inherently prone to misfolding and aggregation into amyloid fibrils, and although they are all structurally and functionally different, they share a characteristic β-sheet structure. In normal physiological conditions, these proteins exist in the cell in a state of equilibrium between properly folded and misfolded forms. Should the balance tip towards misfolding, cells have an internal quality control mechanism — the unfolded protein response — that usually thwarts amyloid formation.
But when the system becomes overwhelmed because of aging or because of genetic mutations in some individuals, pathological protein misfolding can occur. Amyloid fibrils can accumulate in the heart, liver, kidneys, gastrointestinal tract, lungs, muscle skin and neurons, impairing organ function and causing damage and disease. Cardiac ATTR, for instance, was once considered uncommon, but now is thought to occur in 14% of patients with heart failure. In a Finnish study in patients aged over 85, amyloid deposits in the heart were observed in 25% of the participants.
More than 15 different forms of systemic amyloidosis have been identified, all caused by different precursor proteins and leading to amyloid formation and tissue deposition. The clinical symptoms are usually insidious and non-specific, which has made amyloid typing difficult. But recently, distinct amyloid types have been uncovered by applying mass spectrometry-based shotgun proteomics.
Much of the initial success in developing drugs for systemic amyloidosis has occurred in ATTR, which can arise from either sporadic or hereditary misfolding of transthyretin, a tetrameric protein that is made in the liver and secreted into plasma to carry the hormone thyroxine and retinol (vitamin A). They work by blocking the protein’s synthesis, stabilizing transthyretin tetramers or disrupting transthyretin fibrils.
One surprising observation that has galvanized the research community is that some forms of amyloidosis can reverse spontaneously. A group in the UK, Italy and the US, led by Julian Gillmore of University College London (UCL), reported last year that spontaneous recovery from heart failure arising from ATTR-CM was feasible. They identified three patients — from a cohort of 1,663 — who mounted antibody-based immune responses to extracellular deposits of amyloid in heart tissue. Their immune responses not only reversed their symptoms but also restored cardiac structure and function to near normal levels. “Resolution can happen, but it’s extremely rare,” says Laura Sepp-Lorenzino, CSO at Intellia Therapeutics.
Capturing such a potent antibody response in a therapeutic is not easily done. A monoclonal antibody directed against amyloid fibrils is not equivalent to a naturally occurring polyclonal antibody response. No such responses have been reported for the antibody-based therapies for ATTR-CM currently in clinical development. Nevertheless, these intriguing observations have set a target at which antibody developers can aim. Cambridge, UK-based Immutrin is one such firm that has taken inspiration from the study. It’s still an early-stage venture, but its founders, Mark Pepys, emeritus professor of medicine at UCL, and Nobel laureate Greg Winter, of the University of Cambridge, are influential figures in amyloid disease research and in antibody development, respectively. “We have generated antibodies already, and we’re looking to take them to the next stage,” says Immutrin CEO Mihriban Tuna. The expectation is that, for ATTR, potent antibodies will clear amyloid deposits and provide extra benefit on top of that produced by the drugs approved for treating ATTR.
Anti-amyloid agents eliminate toxic deposits by lowering the amount of transthyretin that is available for misfolding and aggregation. They do so by stabilizing the misfolded protein or silencing it. Pfizer’s Vyndaqel (tafamidis), the first ATTR agent approved, is a stabilizer. Vyndaqel, which emerged from the lab of Jeffery Kelly at Scripps Research, works by selectively binding and stabilizing transthyretin in its functional tetrameric state, thereby lowering its rate of dissociation into aggregation-prone monomers. Pfizer acquired the drug in 2010 from FoldRx Pharmaceuticals, which Kelly co-founded with the late Susan Lindquist of MIT, and in 2011 it was approved by the European Union, initially in ATTR with polyneuropathy; in 2019 it was approved by the US Food and Drug Administration. It is now Pfizer’s third-best-selling drug, having racked up almost $2.5 billion in sales in the first half of this year.
Bridge Bio’s Acoramidis is poised to become the second approved transthyretin stabilizer, after data from a phase 3 trial published earlier this year showed that it outperformed placebo on survival, cardiovascular-related hospitalization, changes in N-terminal pro-B-type natriuretic peptide (a biomarker of heart failure) and performance on a six-minute walking test. The 30-month survival rate for trial participants in the active treatment arm was 80.7%, as compared with 74.3% for those in the control arm. The drug mimics the effect of a mutation in the gene encoding transthyretin that leads to a T119M substitution, which results in increased stability of the tetramer and confers a protective effect on heterozygous individuals who have one protective allele and one risk-associated one.
As regards transthyretin silencers, four have so far gained approval. Two are siRNA drugs developed by Alnylam Pharmaceuticals: Onpattro (patisiran) and its more potent successor Amvuttra. Each is approved only in ATTR with polyneuropathy, however, which affects many fewer people than ATTR-CM. Recently published phase 3 data will support a filing for Amvuttra’s approval in the latter indication: it reduced participants’ risk of death or of a cardiovascular event by 28% compared with placebo. Ionis Pharmaceuticals has developed two antisense oligonucleotide-based transthyretin silencers, Wainua (eplontersen) and Tegsedi (inotersen), which Cambridge, UK-based AstraZeneca and Stockholm-based Swedish Orphan Biovitrum are respectively commercializing. Each is approved in ATTR with polyneuropathy only, but a phase 3 trial of Wainua in ATTR-CM is ongoing.
Gene editing company Intellia Therapeutics and its partner Regeneron Pharmaceuticals are also in phase 3 trials in ATTR-CM, with a CRISPR–Cas9-based therapy, nexiguran ziclumeran (‘nex-z’), which targets the TTR locus in liver cells. A phase 3 trial in ATTR with polyneuropathy is due to get underway before the year end. The therapy, which is formulated in lipid nanoparticles and administered in vivo, has more powerful and sustained silencing effects than the currently approved products, which, the company hopes, may translate into superior efficacy. “Once you knock down a gene and you reach nadir, that’s it,” says Sepp-Lorenzino. In non-human primates, this effect was maintained for at least two years. “That gives us confidence the effect is likely to be one and done,” she says.
The therapy can be readministered, should the need arise. Intellia tested this by providing a second, higher dose of nex-z to three participants on a phase 1 dose-escalation study who had originally received a subclinical dose. “The safety was pristine,” says Sepp-Lorenzino. The TTR locus is a particularly attractive target, as there appears to be no unsafe lower limit for the plasma concentration of transthyretin. Knockout animals are healthy, she says, as other proteins can take over its transport functions. As Nature Biotechnology went to press, Intellia was due to unveil updated phase 1 data, which will provide further insights into nex-z’s potential.
Beyond targeting transthyretin, one company, Attralus, is working on a ‘pan-amyloid’ therapeutic and diagnostic. that can bind all forms of amyloid regardless of the misfolded protein species that causes it. The idea is to exploit a striking structural feature common to all amyloid fibrils: regardless of their protein composition, they bind the dye Congo red, which is, in fact, used in histological staining to confirm the presence of amyloid deposits. Company co-founder Jonathan Wall, of the University of Tennessee Graduate School of Medicine, is a veteran of anti-amyloid antibody discovery research — his lab contributed to the early identification of Prothena Biosciences’ birtamimab, which binds a cryptic epitope that is exposed on misfolded κ and λ immunoglobulin light chains. His group subsequently identified a polybasic peptide, p5R, that binds multiple types of amyloid and that forms the basis of its development efforts. “It recognizes a pattern rather than a specific sequence,” says Wall, who is also interim CSO at Attralus.
As well as protein, amyloid deposits also contain glycosaminoglycans (GAGs). “They are highly negatively charged. In fact, they are more like heparin,” he says. These GAGs are distinct from those ubiquitous in every cell and in the extracellular matrix, and they engage in an electrostatic interaction with arginine residues present in the exposed peptide p5R when amyloid is misfolded. Imaging studies have shown that p5R does not bind to GAGs present in healthy tissues.
Attralus plans to use p5R as a diagnostic agent, as well as a therapeutic. The company has received FDA Breakthrough Therapy designation for its p5R-based 124I-evuzamitide (AT-01), intended for positron emission tomography imaging of cardiac amyloidosis. The designation resulted from data obtained in Attralus-sponsored and investigator-initiated studies in over 200 patients with the condition. “Diagnosis of systemic amyloidosis is currently complex — biopsy and Congo red staining are the gold standard, but cardiac biopsies are invasive,” says Glen Firestone, chief business officer at Attralus.
The company’s lead investigational drug, AT-02, comprises a fusion between the same peptide and a humanized IgG1 monoclonal antibody, the Fc region of which is intended to trigger an immune response and amyloid removal. Clinical trials in ATTR amyloidosis and light chain amyloidosis are underway.
Jeffery Kelly, meanwhile, and former FoldRx CEO Richard Labaudinière have co-founded another startup, Protego Biopharma, focused on identifying new molecular chaperones as well as activators of the unfolded protein response to support correct protein folding and trafficking in a range of protein misfolding conditions, including systemic amyloid disease. It has not disclosed details of its specific programs as yet, but it has licensed several activators of the unfolded protein response from Luke Wiseman of Scripps Research, who is a scientific advisor to the compan y.
Amyloid disease can be seen as a part of a larger universe of conditions that involve protein misfolding. As scientists uncover new biological insights, new therapeutic approaches continue to emerge. Scientists in Canada and the Netherlands have recently proposed a prion-like seeding interaction between superoxide dismutase 1 (SOD1) and TAR DNA binding protein (TDP-43) as a pathological mechanism in at least some forms of amyotrophic lateral sclerosis (ALS). “I think the one thing to think about, that makes the experiments challenging, is it might be a fairly transient interaction,” says Ted Allison, of the University of Alberta, who was one of the corresponding authors on the study.
The SOD1 protein appears to be particularly prone to aggregation. Mutations in the gene encoding SOD1, an enzyme that breaks down superoxide radicals, are implicated in one familial form of ALS, which is addressed by Biogen’s antisense drug Qalsody (torfersen). “There are mutations all through SOD1 that cause misfolding,” says Allison. In zebrafish, co-expression of either mutant or wild-type TDP-43 with SOD1 results in neuronal damage arising from SOD1 misfolding. The group also identified a drug repurposing opportunity through in vivo screening in a zebrafish model expressing human SOD1 and TDP-43. Telbivudine, a nucleoside analog, which gained approval as a hepatitis B drug in 2006 but which was withdrawn ten years later for competitive rather than safety reasons, alleviated the SOD1-mediated toxicity in a dose-dependent manner. Patients with ALS who carry a SOD1 mutation may be the most obvious group to recruit into a trial, but the group will perform rodent experiments in advance of any human studies.
In the next couple of years, as tafamidis becomes more widely available as a generic drug for ATTR amyloidosis, it will form a cheap backbone therapy on top of which other agents can be layered. At the same time, a wealth of clinical data from the current slew of pivotal trials will also become available. There is still a long way to go before the prospect of a functional cure for ATTR-CM can be considered a realistic goal. But the field is making valuable progress — and even five years from now, biotechs will find amyloidosis a very different proposition from what it is at present.
Discover more from Tamfis Nigeria Lmited
Subscribe to get the latest posts sent to your email.



 Hot Deals
Hot Deals Shopfinish
Shopfinish Shop
Shop Appliances
Appliances Babies & Kids
Babies & Kids Best Selling
Best Selling Books
Books Consumer Electronics
Consumer Electronics Furniture
Furniture Home & Kitchen
Home & Kitchen Jewelry
Jewelry Luxury & Beauty
Luxury & Beauty Shoes
Shoes Training & Certifications
Training & Certifications Wears & Clothings
Wears & Clothings
















