Technology tamfitronics
Fundamental
Anticoagulants are severely crucial therapies for the prevention or reversal of thrombotic events and characteristic by reducing fibrin deposition by inhibiting fibrinogen proteolysis and/or platelet activation1. A key map of anticoagulant treatment is the protease thrombin (coagulation component IIa (FIIa)). The use of anticoagulants is per a chance–profit prognosis, per which prevention or reduction of progression of thromboembolic disease outweighs the increased chance of bleeding in negative events or trauma. Indeed, many anticoagulants, in particular heparin and warfarin2require shut scientific monitoring to forestall lifestyles-threatening bleeding aspect effects. Nonetheless, anticoagulant-related bleeding and unwanted effects are liable for an estimated 15% of all emergency clinic visits for negative drug effects3 and, as such, programs for the reversal of anticoagulation are due to this of this fact needed4. A total map to reverse the effects of anticoagulants is the administration of nonspecific reversal agents, which entails the infusion of coagulation components designed to overwhelm the effects of circulating anticoagulants5. In scientific settings, unfractionated heparin is a if truth be told useful anticoagulant because protamine sulfate would possibly additionally additionally be feeble for rapid reversal, but unfractionated heparin requires shut monitoring6,7. More now not too long ago, monoclonal antibodies and recombinant FXa had been developed, which bind to particular miniature-molecule anticoagulants with high affinity (idarucizumab for dabigatran and andexanet-α for apixaban, edoxaban and rivaroxaban), thus reversing the inhibition of FXa or thrombin8,9. Despite the incontrovertible fact that these approaches are effective, there are limitations for their use, and so they’re expensive.
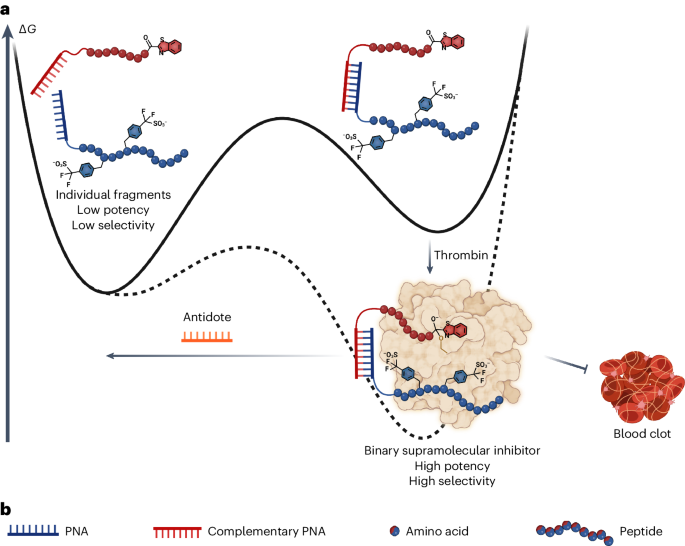
Right here, we most unique a style to generate potent thrombin-inhibiting anticoagulants with on-ask reversibility by programmed supramolecular assembly10. Supramolecular entities rely on labile noncovalent interactions and by their very nature are dynamic and reversible per particular environmental cues or stimuli by intriguing equilibria within the blueprint11. These parts of supramolecular programs had been elegantly utilized to molecular recognition, catalysis, molecular motors, stimuli-responsive polymers and drug discovery and present but, to our data, hold now not been realized for purposes in medicinal chemistry and pharmacology12,13,14,15,16. Our formula is per the ability to hyperlink two fragments by a reversible supramolecular interaction, and these two fragments can engage cooperatively with the map at two particular sites (Fig. 1), with the formation of the stuffed with life inhibitor suggested by the map. Disruption of the supramolecular interaction linking the 2 fragments leads to a loss of cooperativity and thus of inhibitory activity. Our form of supramolecular thrombin inhibitors made use of binary interactions directed to the stuffed with life home of thrombin and to exosite II (the so-called heparin binding home), joined collectively by hybridized peptide nucleic acid (PNA) molecules.
aAssembly of the supramolecular drug is catalyzed by the binding to thrombin, which creates a extremely potent and extremely selective inhibitor from two compounds with low efficiency and selectivity. The inhibition of thrombin would possibly additionally additionally be without warning reversed by the addition of an antidote. ∆GGibbs free vitality exchange. bYarn of ingredients represented in a.
Results
The designed supramolecular assembly became once inspired by thrombin inhibitors produced naturally by blood-feeding (hematophagous) organisms, much like leeches, ticks, mosquitoes and flies, which secrete miniature protein thrombin inhibitors from their salivary glands to facilitate acquisition and digestion of a bloodmeal. These salivary proteins gift potent thrombin inhibition by interacting with two particular binding sites on thrombin, but this activity can’t be without peril reversed due to this of their extraordinarily high affinity for thrombin. At the outset, we intriguing on hyalomin 1, a 59-residue sulfated protein secreted by the tick Hyalomma marginatum rufipes that shares sequence similarity to diversified tick anticoagulant proteins but is truly the most potent thrombin inhibitor within the family (Ki = 5.4 pM). Prognosis of the X-ray crystal constructions of a lot of of these proteins complexed with thrombin (as an illustration, tick-derived madanin-1 (Protein Facts Bank (PDB) 5L6N)17 and tsetse thrombin inhibitor (TTI) from the tsetse hover (PDB 6TKG))18 along with the thrombin inhibitory data suggested that the potent inhibition exhibited by these molecules became once derived from interactions at two loci of thrombin, the stuffed with life home and exosite II, separated by 20–30 Å (Prolonged Facts Fig. 1a–c). We reasoned that lets leverage an established ketobenzothiazole-containing mechanism-essentially based fully mostly pan-serine protease inhibitor for stuffed with life home focusing on that kinds reversible covalent (hemiketal) intermediates with serine proteases but is now not selective for thrombin19. For the peptide focusing on exosite II, we investigated sequences from a lot of salivary proteins from hematophagous organisms that maintain sulfotyrosine residues as a total put up-translational modification that has been confirmed to toughen activity (Prolonged Facts Fig. 1c)17,18,20,21. Pondering the reported lability of the tyrosine sulfate put up-translational modification, we opted for an man made analog of the pure modification, particularly sulfono(difluoro)methyl-phenylalanine (F2Smp)22. For the hyperlink between the 2 binding motifs in our supramolecular anticoagulant, we selected to use the synthetic DNA mimetic PNA23 per the tunability of the hybridization dynamics of this molecular class to offer anticoagulant reversibility, its metabolic steadiness and the compatibility of its chemistry with peptide synthesis24.
Synthesis and characterization of supramolecular inhibitors
We first feeble proper-section synthesis to prepare the mechanism-essentially based fully mostly stuffed with life home focusing on peptide fragment A (A1, derived from hyalomin 1 fused to a ketobenzothiazole warhead) linked to an 8-mer PNA sequence and fragment E (E1, derived from the exosite II binding spot of TTI) linked to the complementary 8-mer PNA (Fig. 2a and Prolonged Facts Fig. 2). Given the identified importance of two native negatively charged sulfotyrosine residues for interaction with the heparin binding exosite II in TTI, we included two F2Smp residues as proper mimics in fragment E1. Fragment A1 showed realistic inhibitory activity against thrombin (Ki = 58.7 nM) in a fluorogenic thrombin activity assay, whereas E1 on my own possessed no inhibitory activity (Fig. 2b). Nonetheless, an 800-fold enhancement of activity became once noticed when both ingredients had been mixed collectively the use of the 8-mer PNA supramolecular connection, with A1–E1 exhibiting a Ki of 74 pM (Fig. 2b). This supramolecular inhibitor additionally received selectivity for thrombin when examined against a panel of proteases most unique within the coagulation pathway, along side FXa, FXIa, FXIIa and plasma kallikrein (PK) (>1,000-fold; Fig. 2c). It is worthy that, like thrombin, the substrate specificities for FXa and FXIa additionally strongly desire arginine at P1 (refs. 25,26), but only thrombin advantages from the bivalent interaction of the supramolecular drug, resulting in greater than 1,000-fold selectivity. To further investigate the supramolecular connectivity between the 2 fragments, we diminished the length of the PNA from 8-mer to 6-mer or 4-mer while conserving the total distance equal. This ended in a revolutionary loss of activity (Fig. 2nd). Nonetheless, the assembly soundless of the shortest supramolecular linker (4-mer: A3–E3) became once aloof tenfold stronger than the stuffed with life home inhibitor on my own (A1). Collectively, these data give a take hold of to a cooperative interplay between the supramolecular interaction of the PNA and the inhibition of thrombin by engagement with both the stuffed with life home and exosite II. The hybridization KD of the 4-mer PNA became once measured by floor plasmon resonance (SPR) to be 4.14 µM at 25 °C (Prolonged Facts Fig. 3), but the supramolecular tether aloof yielded a profit at concentrations neatly below the KD. Cooperativity in inhibition became once noticed if the equilibrium rebinding of the stuffed with life home ligand became once faster within the supramolecular assembly–enzyme complex than the dissociation of the supramolecular tether. It stands to reason that the longer PNA with slower okayoff yields better cooperativity.
aSchematic representation of cooperative dynamic drug assembly. The inhibitors disclosed in this behold are soundless of two fragments: the stuffed with life home-directed fragments, that are numbered A1 to A8, and the exosite II-directed fragments, that are numbered E1 to E23. Mixture of the 2 fragments yields a potent inhibitor named as the combo of the 2 assembled fragments (as an illustration, A1–E1 is the combo of stuffed with life home fragment A1 and exosite II fragment E1). bThrombin inhibition data for the mixed inhibitor versus the 2 fragments on my own. cSelectivity data for A1–E1 against a panel of total proteases. dMake of PNA length on inhibition. eConstruction–activity relationship data of the exosite II binder by diversified payment. fConstruction–activity relationship data of the exosite II binder by diversified hydrophobic amino acids as an different of isoleucine. For all data, n = 3 replicates, with particular person data parts presented as indicate values ± s.d., with the exception of the facts in c where n = 2.
The use of PNA as a supramolecular tether additionally affords the different to rapid assemble analogs and cancel construction–activity studies because contemporary combinations would possibly additionally additionally be generated by simply mixing the binary ligands. We first explored diversified proper sulfotyrosine mimics within the exosite II binding fragment by incorporating disulfonic benzoate (DSB) in lieu of F2Smp (in most cases known as sY herein) into the E fragment to generate E4 (sY12 → DSB), E5 (sY9 → DSB) and E6 (sY9,sY12 → DSB) that would possibly maybe additionally be feeble to invent supramolecular assemblies with stuffed with life home binding fragment A1 by uncomplicated mixing (Fig. 2e)18. Inclusion of DSB in verbalize of F2Smp at verbalize 12 ended in a twofold assemble of activity (A1–E4; Fig. 2e), but alternative of both F2Smp residues with DSB moieties ended in a decrease in inhibition (A1–E6; Fig. 2e). The verbalize and different of sulfotyrosine mimics additionally had a proper affect (A1–E1 versus A1–E7/A1–E8; Fig. 2e). We next performed an alanine scan of the peptide sequence of E1 that focused exosite II. This revealed an isoleucine residue at verbalize 7 as a sizzling spot (Prolonged Facts Fig. 4a), an disclose per the construction of the TTI–thrombin complex (PDB 6TKG; Prolonged Facts Fig. 4b)18which displays this isoleucine filling a hydrophobic pocket. A realistic (roughly twofold) assemble in activity would possibly additionally be completed with substitution for hydrophobic nonproteinogenic amino acids (as an illustration, tert-leucine or norleucine in A1–E15 or A1–E16, respectively; Fig. 2f).
In vitro review of the supramolecular inhibitor
Having established the feasibility of the supramolecular inhibitor belief, we chosen A1–E1 as a lead to profile in subsequent biochemical assays and for anticoagulant activity in vitro. To this conclude, we first investigated the inhibition of fibrinogen proteolysis, whereby A1–E1 exhibited whole inhibition at 100 nM (Prolonged Facts Fig. 5a), whereas A1 or E1 on my own became once same to no inhibitor. Having demonstrated that A1–E1 would possibly maybe prevent fibrinogen proteolysis in vitro, we next grew to alter into our attention to an activated partial thromboplastin time (aPTT) assay in both human and mouse plasma. aPTT assays are routine assessments performed by physicians and are feeble as an indicator of the characteristic of coagulation components within the intrinsic and total pathways. Effective inhibition of thrombin is anticipated to lengthen the time plasma takes to clot, and a clinically critical elevate in clotting is asserted to be twofold. A1–E1 exhibited a therapeutically critical prolongation of clotting time in both human and mouse plasma at a concentration as low as 250 nM (Prolonged Facts Fig. 5b). We next investigated the effects of A1–E1 on thrombin skills in a calibrated computerized thrombogram (CAT). The CAT makes use of a fluorogenic thrombin substrate, thus allowing dimension of thrombin formation in plasma in proper time. Right here is of particular importance because thrombin skills is a dynamic process; the coagulation cascade has many options loops and inhibitory pathways that are all right this moment or now not at once influenced by the increasing thrombin concentration, and thrombin performs a central and pivotal position everywhere in the whole process. Moreover, and in distinction to aPTT assays, the CAT permits for a broad variation within the concentration and persona of the trigger feeble and would possibly maybe due to this of this fact be implemented to detect subtle variations between thrombin inhibitors. A1–E1 potently inhibited thrombin activity in both the initiation section and propagation section of coagulation and became once in an area to fully inhibit thrombin activity at 2.5 µM (Prolonged Facts Fig. 6).
In vivo review of the supramolecular inhibitor
Having decided that our supramolecular anticoagulant potently inhibited thrombin activity and possessed anticoagulant activity in vitro, we next investigated whether or now not A1–E1 would possibly maybe be effective at inhibiting thrombus formation in vivo. To uncover a correct dose for our in vivo efficacy behold, we feeble an ex vivo aPTT assay. Mercurial, A1–E1 became once administered intravenously to mice at 2.5 or 5 mg per kg (body weight), and blood samples had been restful at 5, 15 and forty five min. Clotting occasions had been then measured the use of a frail aPTT protocol and showed that a single bolus (5 mg per kg (body weight)) became once effective at prolonging the aPTT greater than twofold for 30 min (Prolonged Facts Fig. 5c). We next assessed the in vivo efficacy of the supramolecular anticoagulant A1–E1 when when put next with the in style of care argatroban in a localized needle damage model27. This damage leads to both fibrin formation and platelet aggregation in thrombus formation, which had been visualized by Alexa 546-conjugated anti-fibrin and DyLight 649-conjugated anti-GP1bβ28. Owing to its rapid half of-lifestyles in vivo, argatroban became once dosed with an intravenous bolus (3.9 μmol kg–1 (2 mg per kg (body weight))) adopted by an infusion at 24 μmol kg–1 over 60 min (12 mg per kg (body weight), total dose of 27.9 μmol kg–1). A1–E1 became once dosed twice through intravenous bolus at 0.63 μmol kg–1 (5 mg per kg (body weight)) 30 min apart (total dose of 1.3 μmol kg–1). Both A1–E1 and argatroban showed critical decreases in fibrin formation and thrombus dimension (Fig. 3). After medication with the supramolecular anticoagulant A1–E1 adopted by damage, we noticed shut to forestall inhibition of fibrin deposition at the positioning of damage when when put next with govern-treated injuries (Fig. 3). We additionally noticed that A1–E1 completed a same stage of anticoagulation as a bolus infusion of argatroban at the 5 mg per kg (body weight) dosing routine (Fig. 3). On a molarity foundation, A1–E1 yielded similar results to the in style of care (argatroban) at 24-fold decrease drug loading, indicating that the potent inhibitory activity noticed in vitro interprets in vivo.
aTime course of fibrin fluorescence intensity and total thrombus volume (left) and bar chart quantification of average fibrin intensity and average thrombus volume (just) for management animals (n = 7), argatroban-treated animals (n = 4; 2 mg per kg (body weight) bolus adopted by an infusion of 12 mg per kg (body weight)) and A1–E1-treated animals (n = 5; 5 mg per kg (body weight) bolus). Statistical significance between a lot of medication teams became once analyzed the use of an typical one-manner prognosis of variance (ANOVA) with Tukey’s a lot of comparisons checking out with a single pooled variance. For a lot of comparisons checking out, the indicate of every column became once when when put next with the indicate of every diversified column. Facts are presented as indicate ± s.e.m., where ‘n’ equals the different of neutral experiments performed; NS, now not critical (P = 0.6660 for average fibrin intensity and P = 0.5128 for average thrombus volume); **P = 0.0025; ****P bExemplar image of a thrombus 15 min after needle damage without inhibitor (left), with A1–E1 (middle) and with argatroban (just). Platelets are confirmed in crimson, fibrin is confirmed in green, and collagen within the background is confirmed in white; scale bars, 10 μm.
On-ask on/off activity switching in vitro and in vivo
Having established promising in vivo efficacy for our supramolecular inhibitor, we grew to alter into our attention to analyze the ability to reverse the anticoagulant activity with an antidote. Given the non-covalent nature of the supramolecular linker between the stuffed with life home and exosite II binding entities, we rationalized that the inhibition would possibly additionally be disrupted by competing for the hybridization. To desire the equilibrium toward the dissociation of the binary fragments, the competitor PNA became once designed to encompass diaminopurines as an different of adenine because oligomers containing diaminopurines invent more proper duplexes with their complementary strand than oligomers containing adenine29. Despite the incontrovertible fact that this competitor (AD1) functioned as an effective antidote by reversing inhibition, the kinetics of the antidote had been deemed too unhurried at low concentrations (1–10 μM; Prolonged Facts Fig. 7). Mindful of the noticed cooperativity between map interaction and hybridization, we presented a toehold sequence30 on the supramolecular connector (A8–E1) to create an even bigger equilibrium shift within the hybridization with AD2, a 12-mer PNA (Fig. 4a,b). Following the kinetic development of the response in proper time with a fluorogenic substrate, we noticed the ability to change from whole inhibition (15 nM binary inhibitor) to ~40% of uninhibited thrombin activity within 30 min the use of 10 μM antidote (Fig. 4c). The use of decrease concentrations of antidote resulted in more revolutionary restoration of thrombin activity. The use of just 1 equiv. of antidote became once adequate to revive ~20% of thrombin catalytic activity within 90 min. These observations had been additionally validated within the fibrinogen clotting and CAT assays described earlier, with clotting restored the use of 1 equiv. and 5 equiv. of antidote relative to the supramolecular inhibitor, respectively (Fig. 4d, e; A8–E1 + AD2). Consistent with these promising in vitro data, we assessed the ability of our designed antidote to reverse anticoagulation within the in vivo thrombosis model. In this experiment, we first treated animals with our supramolecular invent (5 mg per kg (body weight), the concentration that equipped effective anticoagulation within the needle damage thrombosis model), adopted by administration of 5 molar equiv. of the 12-mer PNA antidote (9.4 mg per kg (body weight)). Following addition of the antidote, anticoagulation became once successfully reversed, as decided by the amount of fibrin deposition and thrombus volume when when put next with govern injuries lacking medication with the antidote (Fig. 4f,g). These data give a take hold of to the aptitude of supramolecular inhibitors as bona fide therapeutic leads and lay the root for focusing on a unfold of therapeutic targets with this style within the long term.
aSchematic representation of antidote addition and reversal of inhibition. bChemical constructions of adenine and diaminopurine forming hydrogen bonds with thymine. cFluorogenic assay data exhibiting the reversal of thrombin inhibition by the addition of diversified concentrations of antidote after 30 min of inhibition. Facts are presented as indicate ± s.d., with n = 3 replicates. dFibrinogen assay data exhibiting the reversal of thrombin inhibition by the addition of antidote (1 equiv.) after 30 min of inhibition. Facts are presented as indicate ± s.d., with n = 3 replicates. eCAT of A8–E1 with and without antidote. Facts are presented as indicate values, with n = 3 replicates. fReasonable fibrin intensity and average thrombus volume for management-treated animals (n = 7), argatroban-treated animals (n = 4; 2 mg per kg (body weight) bolus adopted by an infusion of 12 mg per kg (body weight)), A8–E1-treated animals (n = 3; 5 mg per kg (body weight) bolus) and A8–E1 + AD2-treated animals (n = 3; 5 mg per kg (body weight) + 5 molar equiv. antidote). Statistical significance between a lot of medication teams became once a nalyzed the use of an typical one-manner ANOVA with Tukey’s a lot of comparisons checking out and a single pooled variance. For a lot of comparisons, the indicate of every column became once when when put next with the indicate of every diversified column. Facts are presented as indicate ± s.e.m., where ‘n’ equals the different of neutral experiments performed. The P values for the average thrombus volume data are *P = 0.0412 (management versus A8–E1), P = 0.7759 (NS; management versus A8–E1 + AD2), ***P = 0.0001 (management versus argatroban), **P = 0.0021 (A8–E1 versus A8–E1 + AD2), P = 0.1208 (NS; A8–E1 versus argatroban) and ****P P values for the average fibrin intensity data are P = 0.4545 (NS; management versus A8–E1 + AD2), P = 0.7333 (NS; A8–E1 versus argatroban) and ****P gExemplar image of a thrombus 15 min after needle damage without inhibitor (left), with A8–E1 (middle) and with A8–E1 + AD2 (just). Platelets are confirmed in crimson, fibrin is confirmed in green, and collagen is confirmed in white; scale bars, 10 μm.
Discussion
We hold got designed extremely potent inform bivalent thrombin inhibitors that exclaim an 800-fold assemble in activity relative to particular person fragments by applying the constitutional dynamic properties of supramolecular binary fragments. The supramolecular pairing became once completed with PNA, allowing uncomplicated tuning of the dissociation kinetics of the supramolecular complex. A extraordinarily crucial level of inequity between these molecules and classical inhibitors is that the dynamic equilibrium would possibly additionally additionally be modulated by external components, yielding a straightforward formula for reversing inhibition. This characteristic would possibly maybe be very pertinent for inform thrombin inhibition due to this of the chance of negative bleeding aspect effects in anticoagulation treatment. These identified aspect effects hold stimulated the enchancment of a lot of antidotes to clinically accredited anticoagulant medicines31 that middle on the use of expensive monoclonal antibodies and cocktails of aggressive coagulation proteins. Our designed supramolecular anticoagulants showed potent thrombin inhibition and anticoagulation actions in vitro that would possibly maybe additionally be without warning reversed the use of miniature PNA-essentially based fully mostly antidotes. This potent anticoagulant activity with on-ask reversibility became once additionally demonstrated in an in vivo thrombosis model, providing a starting level for future use of this therapeutic modality for anticoagulant drug candidates. PNAs are identified to be metabolically proper and, unless purposefully modified, cell impermeant32. These parts make PNA a factual chance to focal level the activity of supramolecular medicines on extracellular targets, limiting off-map effects33 and any intrinsic pharmacological activity for the antidote. Future improvements would possibly maybe make use of γ–modified PNA34 with d-stereochemistry to preclude interaction with endogenous extracellular oligonucleotides35,36.
The formula adopted here affords a each day mechanism to flip therapeutic activity on or off without warning and is due to this of this fact now not little to purposes in thrombosis. To illustrate, the supramolecular belief would possibly additionally be critical in immunotherapy when an antidote to a chimeric antigen receptor T cell response is desired or to reverse the action of immunomodulators in case of excessive infection. The incontrovertible fact that assembly would possibly additionally additionally be encoded by diversified sequences of low-payment PNA would possibly additionally aloof make it likely to multiplex programmable supramolecular drug candidates. This formula requires the identification of two fragments that bind synergistically to a protein of ardour. DNA-encoded libraries making use of twin exclaim are poised to snarl such fragments for imprint spanking contemporary targets lacking prior data37,38. The an identical map can additionally be thought to be as with Fab antibody fragments39.
Ideas
Standard programs
Except otherwise specified, all reagents and solvents for all natural synthesis procedures had been bought from industrial sources and had been feeble without further purification. Excessive-performance liquid chromatography (HPLC) purification became once performed with an Agilent Applied sciences 1260 Infinity HPLC the use of a ZORBAX 300SB-C18 column (9.4 × 250 mm). LC–mass spectrometry (LC–MS) spectra had been recorded on a DIONEX Final 3000 UHPLC with a Thermo LCQ Like a flash Mass Spectrometer Machine the use of a PINNACLE DB C18 column (1.9 µm, 50 × 2.1 mm) operated in decided mode. The total LC–MS spectra had been measured by electrospray ionization. Matrix-assisted laser desorption/ionization–time of flight (MALDI–TOF) mass spectra had been measured the use of a Bruker Daltonics Autoflex spectrometer operated in decided mode. Excessive-resolution mass spectra had been bought on a Xevo G2 TOF spectrometer (ionization mode, electrospray ionization decided polarity; mobile section, methanol at 100 µl min–1). Computerized proper-section synthesis became once performed on an Intavis AG Multipep RS instrument.
Synthesis of PNA–peptide conjugates
Resin (5.0 mg) became once swollen in dichloromethane (DCM) for 10 min and washed twice with dimethylformamide (DMF). Iterative cycles of amide coupling (process 1), capping of the resin (process 4) and deprotection of the protective crew (process 2 or process 3) had been performed to synthesize the PNA probes. The compounds had been deprotected and cleaved from the resin the use of process 5 and at final purified by HPLC. Characterization of the PNA–peptide conjugates became once performed the use of MALDI (Bruker Daltonics Autoflex spectrometer with Flex management 3.4 instrument and prognosis with FlexAnalysis 3.4) and/or LC–MS (DIONEX Final 3000 UHPLC with a Thermo LCQ Like a flash Mass Spectrometer Machine the use of a PINNACLE DB C18 column (1.9 µm, 50 × 2.1 mm) with Thermo Xcalibur 2.2.SP1.48 instrument and prognosis with Thermo Xcalibur Qual Browser 2.2.Sp1.48). For MALDI prognosis, 1.0 µl of the sample (in either water or water/acetonitrile (1:1)) became once mixed with 1.0 µl of 2,5-dihydroxybenzoic acid (DHB) matrix solution (30 mg of DHB in 1.0 ml of 70:30:0.01 water/acetonitrile/trifluoroacetic acid (TFA)), and the combination became once noticed on a MALDI plate. The measurements had been obtained in decided linear mode. For LC–MS prognosis, 20 µl of sample in water or water/acetonitrile (1:1) became once injected on the LC and further analyzed by MS in decided mode. Compounds containing the benzene disulfonic acid motif would possibly maybe only be analyzed by LC–MS due to this of fragmentation when analyzed by MALDI.
2-Chlorotrityl chloride resin loading
2-Chlorotrityl chloride resin (1.46 mmol g–1 loading) became once swollen in dry DCM for 30 min, adopted by washing with DCM + 1% N,N-diisopropylethylamine (DIPEA; 3 ml, one time) and DCM (3 ml, ten occasions). An answer of Fmoc-Xaa-OH (0.7 mmol g–1 resin) and DIPEA (4 equiv. relative to resin functionalization) in DCM (closing concentration of 0.125 M amino acid) became once added to the resin, which became once shaken at room temperature for 16 h. The resin became once then washed with DCM (3 ml, five occasions), DMF (3 ml, five occasions) and DCM (3 ml, five occasions). The resin became once then capped through medication with 17:2:1 (vol/vol/vol) DCM/methanol/DIPEA (5 ml) for 40 min at room temperature. The resin became once then washed again with DCM (3 ml, five occasions), DMF (3 ml, five occasions) and DCM (3 ml, five occasions) before further use.
Rink amide resin loading
Nova PEG Rink amide resin (0.44 mmol g–1; Novabiochem) became once swollen in DCM for 10 min and washed twice with DMF. Customary amide coupling (process 1) became once performed, adopted by capping of the resin (process 4). The resin became once then washed again with DCM (3 ml, five occasions), DMF (3 ml, five occasions) and DCM (3 ml, five occasions) before further use.
Amide coupling (process 1)
The corresponding Fmoc-protected PNA monomer40 or amino acid (4 equiv., 0.2 M in N-methylpyrrolidone (NMP)) became once incubated for 5 min with HATU (3.5 equiv., 0.5 M in NMP) and unpleasant solution (1.2 M (4 equiv.) DIPEA and 1.8 M (6.0 equiv.) 2,6-lutidine in NMP). The mix became once then added to the corresponding resin. After 20 min, the combination became once filtered, the resin became once washed with DMF, and a brand contemporary premixed response solution became once added to the resin and allowed to react for but another 20 min. The resin became once then washed sequentially with DMF, DCM and DMF two occasions every.
Fmoc deprotection (process 2)
An answer of 20% (vol/vol) piperidine in DMF became once added to the resin and allowed to react for 5 min. The mix became once then filtered, the resin became once washed with DMF, and the sequence became once repeated for but another 5 min. The resin became once then washed sequentially with DMF, DCM and DMF two occasions every.
4-Methyltrityl deprotection (process 3)
An answer (produced from 244 mg of hydroxybenzotriazole in 10 ml of hexafluoroisopropanol and 10 ml of 1,2-dichloroethane) became once added to the prewashed resin to reach a volume of 10 ml g–1 of resin and allowed to react for 5 min. The answer became once flushed, the resin became once washed with DCM, and the sequence became once repeated for but another 5 min. Within the kill, the resin became once washed sequentially with DCM and DMF two occasions every.
Capping (process 4)
The resin became once treated with a capping mixture (0.92 ml of acetic anhydride and 1.3 ml of 2,6-lutidine in 18 ml of DMF; 10 ml of solution per g of resin) for 5 min. After flushing the solution, the resin became once washed sequentially with DMF, DCM and DMF two occasions every.
Cleavage from the resin and closing deprotection (process 5)
Resin (5.0 mg, 1.0 μmol) became once treated with 125 μl of a mix of TFA and scavengers (440 µl of TFA + 25 mg of phenol + 25 µl of water + 10 µl of triisopropylsilane) for 2 h. The resin became once filtered and washed with TFA (50 μl), and the restful fractions of cleavage product had been precipitated in icy ether (1.5 ml). After centrifugation, the pellet became once vortexed again with icy diethyl ether (1.5 ml) and centrifuged (18,000g). The pellet became once dissolved in water/acetonitrile (3:1; 1.5 ml) and lyophilized to create a white powder.
Microcleavage for quality management (process 6)
The minimal different of beads became once picked with a pipette plastic tip and transferred to 50 µl of TFA. The answer became once left for 1 h and transferred to 1.0 ml of ether. The ether solution became once maintained at −20 °C for 5 min and centrifuged for 5 min at 18,000g. The ether supernatant became once removed, and t he pellet became once dissolved in 20 µl of 1:1 acetonitrile/water, which became once then feeble for prognosis by MALDI–TOF and/or LC–MS.
On-resin copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC; process 7)
An answer of CuSO4 (15 µl, 64 mg ml–1 in water) became once added to tris(benzyltriazolylmethyl)amine (2 mg) in 20 µl of DMF, adopted by the addition of fifty µl of sodium ascorbate (396 mg ml–1 in water). Azide-containing peptide (2 equiv. in 60 µl of DMF) became once added to the combination, which became once mixed before the addition of 5 mg of alkyne-derivatized Rink amide resin (0.0022 mmol). After 16 h of shaking, the combination became once filtered, and the resin became once washed six occasions with 250 µl of sodium diethyl dithiocarbamate (0.02 M) in DMF, six occasions in 250 µl of DMF, six occasions in methanol and 6 occasions in DCM.
Coupling of Fmoc-l-F2Smp(nP)-OH (process 8)
Fmoc-l-F2Smp(nP)-OH became once ready as previously described22. A mix of Fmoc-l-F2Smp(nP)-OH (0.003 mmol, 1.5 equiv.), hydroxybenzotriazole (0.003 mmol, 1.5 equiv.) and N,N′-diisopropylcarbodiimide (0.003 mmol, 1.5 equiv.) became once added to the corresponding resin and shaken in a single day. The mix became once filtered, and the resin became once washed sequentially with DMF, DCM and DMF two occasions every.
Coupling of Arg(Pbf)-benzothiazole (process 9)
Arg(Pbf)-benzothiazole (0.0044 mmol, 2 equiv.) and HATU (0.0034 mmol, 1.5 equiv.) in NMP (100 μl) had been added to 5 mg of resin (0.0022 mmol), adopted by the addition of DIPEA (0.012 mmol, 6 equiv.). The response became once shaken for 2 h, the combination became once filtered, and the resin became once washed sequentially with DMF, DCM and DMF two occasions every.
Neopentyl deprotection and characterization of PNA–peptide conjugates (process 10)
The precipitate restful after cleavage and ether precipitation became once lyophilized. The final proper became once dissolved in an answer of 1 M ammonium acetate and 6 M guanidinium chloride and shaken at 37 °C for 2 h. The answer became once then diluted with water/acetonitrile (50:50) and purified by HPLC.
Thrombin inhibition assay
Inhibition of the activity of human α-thrombin (Haematologic Applied sciences, HCT0020) became once adopted spectrophotometrically the use of Phe-Expert-Arg-Coumarin (synthesis described in Supplementary Facts) as the chromogenic substrate.
Inhibition assays had been performed the use of 0.2 nM enzyme, 20 μM substrate and extending concentrations of inhibitor. The concentration of every inhibitor variant became once resolute the use of the absorbance of the PNA at 260 nm, as measured by NanoDrop. All reactions had been performed at 37 °C in 50 mM Tris-HCl (pH 8.0), 50 mM NaCl and 1 mg ml–1 bovine serum albumin in murky 96-neatly microtiter plates (Thermo Fisher Scientific, 267342). Response development became once monitored by excitation at 339 nm and emission at 439 nm the use of a SpectraMax or Tecan Spark Plate Reader. Dose–response curves had been feeble to search out out the half of-maximal inhibitory concentrations (IC50) the use of Prism 8.0 (GraphPad Tool). For every inhibitor, the reactions had been performed in triplicate, along with management reactions within the absence of enzyme. The preliminary mosey became once calculated from the slope of the predominant 10 min of the assay. The curves had been normalized to the neatly without inhibitor, where the preliminary mosey became once spot to 100% activity.
For the antidote assay, the plate became once removed from the plate reader at the specified time of addition (in most cases 30 min). One microliter of antidote (100×) became once added, and reading became once resumed.
Fibrinogen assay
Human α-thrombin (Haematologic Applied sciences, HCT0020; closing concentration of 2.5 nM) became once incubated with compound (closing concentration of 15 nM) at 37 °C for 30 min. Fibrinogen (closing concentration of 1 mg ml–1) became once added, and absorbance at 288 nm became once measured the use of a SpectraMax Plate Reader. All reactions had been performed at 37 °C in 50 mM Tris-HCl (pH 8.0), 50 mM NaCl and 1 mg ml–1 bovine serum albumin in certain 96-neatly microtiter plates (Greiner Bio-One, 650201).
For the antidote assay, the plate became once removed from the plate reader at the specified time of addition (in most cases 30 min). One microliter of antidote (100×) became once added, and reading became once resumed.
Selectivity assays
Inhibitory activity of A1–E1 became once examined against α-human thrombin, FXIa and FXa (Haematologic Applied sciences) and α-FXIIa and PK (Enzyme Study Laboratories). Chromogenic assays had been adopted spectrophotometrically the use of the following particular substrates: 100 μM Tos-Gly-Expert-Arg-PNA (Chromozym TH, Roche) for thrombin, 500 µM Pyr-Expert-Arg-PNA (L-2145, Bachem) for FXIa, 500 µM Moc-d-norleucine-Gly-Arg-PNA (L-1565, Bachem) for FXa and 200 µM or 400 µM d-Expert-Phe-Arg-PNA (Cayman Chemical) for α-FXIIa or PK, respectively. The assay buffers included 50 mM Tris-HCl (pH 8.0) and 50 mM NaCl for thrombin (0.2 nM); PBS (pH 7.4) for FXIa (0.5 nM); 25 mM Tris-HCl (pH 7.5), 100 mM NaCl and 5 mM CaCl2 for FXa (0.5 nM); 20 mM HEPES (pH 7.6), 150 mM NaCl, 0.1% (wt/vol) PEG 8000 and zero.01% (vol/vol) Triton X-100 for α-FXIIa (4 nM); and 50 mM Tris-HCl (pH 8.0) and 150 mM NaCl for PK (0.25 nM). Bovine serum albumin (Sigma) became once added to all buffers at 1 g l–1. All reactions had been initiated by the addition of the protease and had been performed at 37 °C in 96-neatly, flat-bottom microtiter plates. Response development became once monitored at 405 nm for 30 min (60 min for FXa and α-FXIIa) on a multimode microplate reader (Synergy2, BioTek) with measurements taken every 5 min. All measurements had been performed in duplicate. IC50 values had been decided from the log dose–response curves with Prism 9 (GraphPad Tool).
SPR experiments
SPR experiments had been performed on a Biacore T200 instrument (GE Healthcare) at 25 °C in PBS-P+ buffer (10× stock; Cytiva Existence Sciences, 28995084). Biotin-PNA (8-mer) became once immobilized on a Streptavidin Series S sensor chip (Cytiva Existence Sciences, 29104992). Sooner than immobilization, the 2 channels had been conditioned with 1 M NaCl in 50 mM NaOH. After stabilization, the compound (solution in PBS-P+) became once flowed over thought to be one of the waft cells of the sensor chip at a concentration of fifty nM at a waft payment of 10 μl min−1 with a response unit map of 500. Biotin-PNA (8-mer) reached a response unit price of 513.7. The blueprint (now not along side the waft cells) became once washed with 50% isopropanol in 1 M NaCl and 50 mM NaOH after every ligand injection. Kinetic measurements consisted of injections (association, 400 s; dissociation, 450 s; waft payment, 30 μl min−1) of reducing concentrations of PNA (4-, 6- and eight-mer; twofold cascade dilutions from the starting concentration). The chip became once regenerated between cycles by one injection of regeneration solution (50 mM NaOH) for 10 s at a waft payment of 20 μl min−1adopted by a 10-s stabilization length. Binding became once measured as resonance units over time after smooth subtraction, and the facts had been interpreted the use of Biacore T200 instrument (version 3.2). All measurements had been performed in duplicate. The KD values had been calculated per in style-verbalize affinity (1:1 binding).
aPTT in vitro
aPTT measurements had been performed on a BFT II benchtop analyzer as per the manufacturer’s directions. Dade Actin FSL Activated PTT Reagent (23-044-647) and calcium chloride solution (10446232 ORHO37) had been both sourced from Siemens Healthcare Diagnostics Products, and lyophilized pooled human reference plasma (Pooled Norm., 00539) became once bought from Diagnostica Stago. Pooled human plasma became once reconstituted as per the manufacturer’s directions (Milli-Q water, 30 min, room temperature). Pooled mouse plasma became once ready by assortment of whole blood from three to four C57BL/6 mice (Australian BioResources) into sodium citrate (3.8%), with plasma remoted by centrifugation at 5,000g for 15 min and kept on ice till required.
Human or mouse plasma became once incubated with inhibitors at the indicated concentrations and prewarmed to 37 °C. Fifty microliters of every plasma/inhibitor mixture became once incubated with Actin FSL (50 ml) in a stirred response vessel for 3 min before addition of fifty ml of calcium chloride solution to originate coagulation. The time taken for fibrin clot formation became once recorded in a semiautomated style the use of a BFT II Analyzer, which makes use of a turbodensitometric detection methodology.
Ex vivo aPTT
All procedures energetic the use of animals had been performed as accredited by the University of Sydney Animal Ethics Committee (protocol 2021/1912). C57BL/6 mice (25–30 g) had been anesthetized the use of a mix of ketamine (125 mg per kg (body weight)) and xylazine (12.5 mg per kg (body weight); intraperitoneal offer) and administered A1–E1 as a single bolus delivered intravenously through the femoral vein at either 2.5 or 5.0 mg per kg (body weight). Blood became once drawn from the negative vena cava at the indicated occasions into citrate anticoagulant (3.8%), plasma became once remoted as described above for in vitro aPTT studies, and aPTT became once assessed through changes in plasma opacity at 405 nm the use of a CLARIOstar plate reader fitted with twin injectors heated to 37 °C the use of a modified version of the aPTT protocol described above. Mercurial, injectors had been primed for Dade Actin FSL Activated PTT Reagent (line A) and calcium chloride solution (line B), and mouse plasma became once aliquoted in duplicate (25 μl) into wells of a Nunc 368-neatly polystyrene plate (Z723010, Sigma-Aldrich). Following injection of 25 ml of Dade Actin FSL, the plate became once mixed the use of the orbital shaking characteristic for 2 s (500 rpm) and incubated for 182 s at 37 °C. Right now (designated t = 0 s), 25 ml of calcium chloride solution became once injected, the plate became once mixed as described above, and absorbance measurements had been taken at 405 nm for 360 intervals (22 flashes per neatly, interval time of 0.5 s). Clotting time became once denoted by the timing of preliminary inflection level, denoting transition of plasma from transparent to opaque.
CAT
Customary lyophilized human pooled plasma (Pool Norm., 00539, Diagnostica Stago) became once reconstituted and incubated for 30 min at 37 °C. Vehicle and various inhibitors at diversified concentrations had been then incubated in plasma for 30 min. Thrombin assays had been performed the use of a Hemker Calibrated Computerized Thrombinoscope (Diagnostica Stago) and a Fluoroskan Ascent plate reader (Thermo Fisher Scientific). All experiments had been performed in triplicate in 96-neatly microplates for fluorescence-essentially based fully mostly assays (M33089, Thermo Fisher Scientific) and calibrated the use of untreated plasma and a thrombin calibrator (86192, Diagnostica Stago). Thrombinoscope experiments had been performed following patented industrial protocols. In rapid, every sample neatly became once stuffed with 20 μl of PPP reagent containing a mix of phospholipids and tissue component (86193, Diagnostica Stago). Eighty microliters of plasma (untreated/ treated) became once then added to every of these wells and mixed the use of reverse pipetting, and the neatly plate became once incubated within the plate reader at 37 °C for 10 min. Within the meantime, a FluCa kit (86197, Diagnostica Stago) containing Fluo-Buffer and Fluo-Substrate became once warmed to 37 °C. Following incubation, the thrombinoscope dispenser became once flushed, emptied and stuffed with a FluCa mixture consisting of the Fluo-Buffer and Fluo-Substrate. Twenty microliters of the FluCa mixture became once disbursed into every neatly containing plasma samples, initiating the coagulation response. Thrombin activity (nM) became once measured over 1 h, with thrombogram parameters along side mosey time (min), mosey index (nM min–1), time to height (min), height height (nM), endogenous thrombin doable (nM × min) and time to tail (min).
Needle damage thrombosis model
C57BL/6J mice had been bought from Australian BioResources and housed at the Laboratory Animal Companies facility (University of Sydney). All animals had been maintained on a 12-h light/12-h murky cycle with receive entry to to food and water ad libitum. For intravital mouse studies, male mice feeble between 5 and eight weeks outdated skool (15–20 g) had been feeble. All studies had been accredited by the University of Sydney Animal Ethics Committee (protocol 2021/1912) per the necessities of the Australian Code of Note for the Care and Employ of Animals for Scientific Purposes41.
A scientific preparation of argatroban (Argatra/Exembol) became once bought from Mitsubishi Tanabe Pharma (Germany) and ready in sterile saline with 25% (vol/vol) propylene glycol. Synthesized PNA inhibitors and PNA inhibitors + antidote solutions had been ready in sterile saline at a concentration of 2 mg ml–1. C57BL/6J male mice (15–25 g) had been anesthetized with ketamine (150 mg per kg (body weight)) and xylazine (15 mg per kg (body weight)), supplemented with oxygen and subjected to intravital needle damage, as previously described42. Systemic injection of DyLight 649-conjugated anti-GP1bβ (X649, Emfret; 100 µg kg–1) and Alexa 546-conjugated anti-fibrin (0.31 mg per kg (body weight)) became once performed before vessel damage to video display thrombus formation and fibrin skills, respectively. Argatroban (80 µg kg–1 bolus, 40 µg kg–1 min–160-min infusion) became once delivered through a jugular catheter the use of a Harvard apparatus pump (704504, Pump 11 Elite I/W Single Syringe Pump). Injections of PNA inhibitors or PNA inhibitors + antidote (5 mg per kg (body weight) bolus every 30 min) had been delivered intravenously. Two to four successive injuries had been created in a lot of vessels in every mouse from every medication crew. Following every damage, platelet thrombus formation and fibrin skills had been monitored over a 15-min length the use of a confocal intravital microscopy platform (Nikon A1R-Si with an Apo LWD, ×40/1.15-NA water immersion map; sequential excitation: 488-, 561- and 638-nm lasers; emission: 525/50-, 595/50- and 700/75-nm filters) and NIS Parts Advanced Study acquisition instrument. The microscope stage and map had been maintained at 37 °C everywhere in the experiment through a Peltier heater (OkoLab). Floor renders of confocal stacks representing thrombi from separate teams had been generated the use of Imaris (version 9.8, Bitplane).
Quantitative prognosis of thrombus volume over time
NIS Parts instrument (version. 5.02; Nikon) became once feeble to apply a threshold to DyLight 649-conjugated anti-GP1bβ signal for every xyz stack in a time series and became once feeble to calculate the quantity for on every occasion level.
Quantitation of exchange in fibrin amount over time
The signal bought from DyLight 649-conjugated anti-GP1bβ for every xyz stack in a time series became once thresholded to make a cover. The total signal (arbitrary units) from Alexa Fluor 546-conjugated anti-fibrin within this cover (that is, the fibrin signal internal the thrombus) for on every occasion level became once then quantified the use of NIS Parts instrument (Nikon).
Statistical prognosis
Statistical significance between a lot of medication teams became once analyzed the use of a one-manner ANOVA with Tukey’s put up hoc checking out with a single pooled variance (Prism instrument version 10.2; GraphPad Tool for Science). Facts are presented as indicate ± s.e.m., where ‘n’ equals the different of neutral experiments performed.
Reporting summary
Extra data on look at form is on hand within the Nature Portfolio Reporting Summary linked to this text.
Facts availability
The facts supporting the findings of this behold are on hand within this paper and its Supplementary Facts. All raw data had been deposited on Zenodo (https://doi.org/10.5281/zenodo.10473739)43. Previously published PDB ID numbers that are mentioned and confirmed within the predominant text would possibly additionally additionally be learned online with the following codes: tick-derived madanin-1 (PDB 5L6N) and TTI from the tsetse hover (PDB 6TKG). Source data are equipped with this paper.
References
-
Di Nisio, M., Middeldorp, S. & Buller, H. R. Drug treatment—inform thrombin inhibitors. N. Engl. J. Med. 3531028–1040 (2005).
-
Hirsh, J., Anand, S. S., Halperin, J. L. & Fuster, V. Facts to anticoagulant treatment: Heparin: a press launch for healthcare professionals from the American Heart Association. Circulation 1032994–3018 (2001).
-
Geller, A. I. et al. Emergency visits for oral anticoagulant bleeding. J.Gen. Internal. With. 35371–373 (2020).
-
Thomas, S. & Makris, M. The reversal of anticoagulation in scientific observe. Clin. Med. 18314–319 (2018).
-
Smith, M. N., Deloney, L., Carter, C., Weant, K. A. & Eriksson, E. A. Security, efficacy, and price of four-component prothrombin complex listen (4F-PCC) in sufferers with component Xa inhibitor-related bleeding: a retrospective behold. J. Thromb. Thrombolysis 48250–255 (2019).
-
Guerrini, M. et al. Oversulfated chondroitin sulfate is a contaminant in heparin related to negative scientific events. Nat. Biotechnol. 26669–675 (2008).
-
Eikelboom, J. W. & Hirsh, J. Monitoring unfractionated heparin with the aPTT: time for a novel look at. Thromb. Haemost. 96547–552 (2006).
-
Pollack, C. V. et al. Idarucizumab for dabigatran reversal. N. Engl. J. Med. 373511–520 (2015).
-
Lu, G. M. et al. A particular antidote for reversal of anticoagulation by inform and indirect inhibitors of coagulation component Xa. Night. With. 19446–451 (2013).
-
Lehn, J. M. Perspectives in supramolecular chemistry—from molecular recognition towards molecular data-processing and self-group. Angew. Chem. Int. Ed. 291304–1319 (1990).
-
Lehn, J. M. Against self-group and advanced topic. Science 2952400–2403 (2002).
-
Ramström, O. & Lehn, J.-M. Drug discovery by dynamic combinatorial libraries. Nat. Rev. Drug Discov. 126–36 (2002).
-
Corbett, P. T. et al. Dynamic combinatorial chemistry. Chem. Rev. 1063652–3711 (2006).
-
Browne, W. R. & Feringa, B. L. Making molecular machines work. Nat. Nanotechnol. 125–35 (2006).
-
Li, J. W., Nowak, P. & Otto, S. Dynamic combinatorial libraries: from exploring molecular recognition to programs chemistry. J. Am. Chem. Soc. 1359222–9239 (2013).
-
Webber, M. J. & Langer, R. Drug offer by supramolecular form. Chem. Soc. Rev. 466600–6620 (2017).
-
Thompson, R. E. et al. Tyrosine sulfation modulates activity of tick-derived thrombin inhibitors. Nat. Chem. 9909–917 (2017).
-
Calisto, B. M. et al. Sulfotyrosine-mediated recognition of human thrombin by a tsetse hover anticoagulant mimics physiological substrates. Cell Chem. Biol. 2826–33.e8 (2021).
-
Costanzo, M. J. et al. In-depth behold of tripeptide-essentially based fully mostly α-ketoheterocycles as inhibitors of thrombin. Effective utilization of the S1′ subsite and its implications to construction-essentially based fully mostly drug form. J. Med. Chem. 505868 (2007).
-
Watson, E. E. et al. Fast assembly and profiling of an anticoagulant sulfoprotein library. Proc. Natl Acad. Know USA 11613873–13878 (2019).
-
Ripoll-Rozada , J. , Maxwell , JWC , Payne , RJ & Pereira , PJB Tyrosine-O-sulfation is a frequent affinity enhancer amongst thrombin interactors. Biochem. Soc. Trans. 50387–401 (2022).
-
Dowman, L. J. et al. Synthesis and review of peptidic thrombin inhibitors bearing acid-proper sulfotyrosine analogues. Chem. Commun. 5710923–10926 (2021).
-
Egholm, M. et al. PNA hybridizes to complementary oligonucleotides obeying the Watson–Crick hydrogen-bonding tips. Nature 365566–568 (1993).
-
Barluenga, S. & Winssinger, N. PNA as a biosupramolecular imprint for programmable assemblies and reactions. Acc. Chem. Res. 481319–1331 (2015).
-
Gosalia, D. N., Salisbury, C. M., Ellman, J. A. & Diamond, S. L. Excessive throughput substrate specificity profiling of serine and cysteine proteases the use of solution-section fluorogenic peptide microarrays. Mol. Cell. Proteomics 4626–636 (2005).
-
Dementiev, A. et al. Structures of human plasma β-component XIIa cocrystallized with potent inhibitors. Blood Adv. 2549–558 (2018).
-
Watson, E. E. et al. Mosquito-derived anophelin sulfoproteins are potent antithrombotics. ACS Cent. Sci. 4468–476 (2018).
-
Kaplan, Z. S. et al. Thrombin-dependent intravascular leukocyte trafficking regulated by fibrin and the platelet receptors GPIb and PAR4. Nat. Common. 67835 (2015).
-
Haaima, G., Hansen, H. F., Christensen, L., Dahl, O. & Nielsen, P. E. Elevated DNA binding and sequence discrimination of PNA oligomers containing 2,6-diaminopurine. Nuc. Acids Res. 254639–4643 (1997).
-
Yurke, B., Turberfield, A. J., Mills, A. P., Simmel, F. C. & Neumann, J. L. A DNA-fuelled molecular machine made of DNA. Nature 406605–608 (2000).
-
Ebright, J. & Mousa, S. A. Oral anticoagulants and space of antidotes for the reversal of bleeding chance. Clin. Appl. Thromb. Hemost. 21105–114 (2015).
-
Saarbach, J., Sabale, P. M. & Winssinger, N. Peptide nucleic acid (PNA) and its purposes in chemical biology, diagnostics, and therapeutics. Curr. Opin. Chem. Biol. 52112–124 (2019).
-
Zhang, Z. Y. et al. Brain-restricted mTOR inhibition with binary pharmacology. Nature 609822–828 (2022).
-
Dragulescu-Andrasi, A. et al. A truly easy γ-spine modification preorganizes peptide nucleic acid exact into a helical construction. J. Am. Chem. Soc. 12810258–10267 (2006).
-
Flynn, R. A. et al. Small RNAs are modified with N-glycans and displayed on the bottom of living cells. Cell 1843109–3124 (2021).
-
Rasmussen, M. et al. RNA profiles expose signatures of future neatly being and disease in pregnancy. Nature 601422–427 (2022).
-
Wichert, M. et al. Dual-exclaim of miniature molecules enables the invention of ligand pairs and facilitates affinity maturation. Nat. Chem. 7241–249 (2015).
-
Vummidi, B. R. et al. A mating mechanism to generate diversity for the Darwinian different of DNA-encoded synthetic molecules. Nat. Chem. 14141–152 (2022).
-
Kazane, S. A. et al. Self-assembled antibody multimers by peptide nucleic acid conjugation. J. Am. Chem. Soc. 135340–346 (2013).
-
Pothukanuri, S., Pianowski, Z. & Winssinger, N. Growing the scope and orthogonality of PNA synthesis. Eur. J. Org. Chem. 20083141–3148 (2008).
-
Nationwide Properly being and Mecial Study Council. Australian Code for the Care and Employ of Animals for Scientific Purposes Eighth edn (NHMRC, 2013).
-
Agten, S. M. et al. Potent trivalent inhibitors of thrombin by hybridization of salivary sulfopeptides from hematophagous arthropods. Angew. Chem. Int. Ed. 605348–5356 (2021).
-
Dockerill, M. et al. Pattern of supramolecular anticoagulants with on-ask reversibility. Zenodo https://doi.org/10.5281/zenodo.10473739 (2024).
Acknowledgements
This work became once supported, in section, by the Swiss Nationwide Science Foundation (219316; to N.W.), NCCR Chemical Biology (185898; to N.W.), the Nationwide Properly being and Medical Study Council of Australia (APP1174941; to R.J.P.) and the Portuguese nationwide funds through Fundação para a Ciência e a Tecnologia by venture PTDC/BIA-BQM/2494/2020 (to J.R.-R. and P.J.B.P.). J.R.-R. additionally acknowledges the give a take hold of to of RYC2021-033063-I funded by MCIN/AEI/10.13039/501100011033 and the European Union NextGenerationEU/PRTR.
Funding
Begin receive entry to funding equipped by University of Geneva.
Ethics declarations
Competing pursuits
The authors speak no competing pursuits.
Recognize review
Recognize review data
Nature Biotechnology thanks the nameless reviewers for their contribution to the peep review of this work.
Extra data
Publisher’s gift Springer Nature remains neutral in regards to jurisdictional claims in published maps and institutional affiliations.
Prolonged data
Prolonged Facts Fig. 1 Amino acid sequence alignment of inhibitors from a unfold of blood-feeding arthropods alongside the supramolecular drug.
a. Schematic representation of sequences of Madanin1 (Mad1), Hyalomin1 (Hya1) and tsetse hover thrombin inhibitors (TTI) alongside A1-E1. b. Crystal construction of the tsetse thrombin inhibitor:thrombin complex (PDB 6TKG) exhibiting the gap between the stuffed with life home and exosite II binding ingredients. c. Full sequences aligned. The exosite II sequence alignment is confirmed on a blue background with the sulfated tyrosine residues highlighted in crimson. The stuffed with life home sequence alignment is confirmed on a crimson background with the scissile bond indicated by an arrow. The PNA is written in italics and the benzothiazole reversible covalent warhead is shortened to ‘BT’.
Prolonged Facts Fig. 2 Chemical Structures of predominant compounds of the behold.
a. Packed with life home-directed inhibitor A1, b. Exosite II-directed inhibitor E1, c. Packed with life home-directed inhibitor with 4-mer toehold PNA A8, d. 8-mer antidote AD1 and e. 12-mer antidote AD2.
Prolonged Facts Fig. 3 KD of binding between PNAs of diversified lengths.
a. Inhibition data for inhibitors with diversified length of PNA. b. SPR data for the binding between 4-, 6-, and eight-mer PNA strands with ACAACTGC immobilised through a biotin on a streptavidin coated SPR chip. The PNA sequences are written N to C, with serine-modified monomers underlined. c. SPR kinetic curves. For data presented in a. and b., n=3 replicates with particular person data parts presented as indicate values +/− SD.
Prolonged Facts Fig. 4 Extra SAR.
a. Exosite II Ala scan – fluorogenic inhibition assay data for A1 mixed with E17 to E22. b. Crystal construction (PDB 6TKG) of the tsetse thrombin inhibitor (TTI) complexed with thrombin. The zoom displays the hydrophobic pocket throughout which isoleucine is found. c. IC50 values for stuffed with life home binders with changes within the P3 positions. d. Comparability between inhibitors inspired from the exosite II sequence of the tsetse thrombin inhibitor (TTI) and Madanin1 (Mad1, from the Haemaphysalis longicornis species) at 15 nM. For data presented in a., c. and d., n=3 replicates with particular person data parts presented as indicate values +/− SD.
Prolonged Facts Fig. 5 Extra Assays.
a. Fibrinogen inhibition assay of compounds A1-E1 and A1 and E1 on my own. b. In vitro aPTT of A1-E1 in human (prime) and mouse (bottom) plasma. n=2 with both replicates displayed. c. Ex-vivo aPTT of A1-E1 at 0.314 μmol/kg (2.5 mg/kg) and zero.627 μmol/kg (5 mg/kg) versus Argatroban at 1.966 μmol/kg (1 mg/kg). For data presented in a., and c., n=3 replicates with particular person data parts presented as indicate values +/− SD.
Prolonged Facts Fig. 6 Calibrated Computerized Thrombogram (CAT).
a. A1, E1, AD1and AD2 examined on my own at a unfold of concentrations. b. Mixed inhibitors A1-E1 and A8-E1 examined at a unfold of concentrations. c. Mixed inhibitors with antidote A1-E1+AD1 and A8-E1+AD2 examined at 2.5 μM with a unfold of antidote concentrations. For all data presented, n=3 replicates with particular person data parts presented as indicate values +/− SD.
Prolonged Facts Fig. 7 Reversal of thrombin inhibition with an 8 mer PNA antidote.
a. Schematic representation of antidote addition and reversal of inhibition. b. Fluorogenic assay data exhibiting the reversal of thrombin inhibition by addition of diversified concentrations of antidote (AD1) after 30 minutes of inhibition. c. Calibrated Computerized Thrombogram (CAT) of A1-E1 with and without antidote (AD1). For data presented in b., and c., n=3 replicates with particular person data parts presented as indicate values +/− SD.
Supplementary data
Supplementary Facts
Supplementary Figs. 1–6, Tables 1 and a pair of, synthesis and characterization of Arg(Pbf)-benzothiazole, synthesis and characterization of Phe-Expert-Arg-Coumarin, characterization of PNA–peptide compounds, thrombin inhibition assay programs, and needle damage thrombosis model maximum intensity projections.
Reporting Summary
Supplementary Facts
Raw photography of maximum intensity projections.
Source data
Rights and permissions
Begin Rep admission to This article is licensed below a Ingenious Commons Attribution 4.0 World License, which permits use, sharing, adaptation, distribution and reproduction in any medium or layout, so long as you give acceptable credit to the distinctive author(s) and the offer, present a hyperlink to the Ingenious Commons licence, and gift if changes had been made. The pictures or diversified third occasion fabric in this text are included within the article’s Ingenious Commons licence, unless indicated otherwise in a credit line to the material. If fabric is now not included within the article’s Ingenious Commons licence and your intended use is now not accredited by statutory laws or exceeds the accredited use, you would possibly maybe have to create permission right this moment from the copyright holder. To explore a reproduction of this licence, seek recommendation from http://creativecommons.org/licenses/by/4.0/.
About this text
Cite this text
Dockerill, M., Ford, D.J., Angerani, S. et al. Pattern of supramolecular anticoagulants with on-ask reversibility. Nat Biotechnol (2024). https://doi.org/10.1038/s41587-024-02209-z
-
Bought:
-
Well-liked:
-
Printed:
-
DOI: https://doi.org/10.1038/s41587-024-02209-z