Health News tamfitronics
The FDA never inspected Johns Dental Laboratories at some stage in more than a decade whereby it made the Anterior Growth Steering Appliance, or “AGGA,” a dental tool that has allegedly harmed sufferers and is now the discipline of a felony investigation.
Per FDA paperwork purchased thru the Freedom of Records Act, the company “grew to alter into conscious” of the AGGA from a joint investigation by KFF Health News and CBS News in March 2023, then answered with its first-ever inspection of Johns Dental months later.
That inspection chanced on that the Indiana dental tool manufacturer didn’t require all customer complaints to be investigated and the corporate did no longer study some complaints about folks being ache by merchandise, alongside side the AGGA, the FDA paperwork mumble. The FDA requires tool companies to study complaints and forward them to the company. Johns Dental had “never” alerted the FDA to any such complaints, in step with the paperwork.
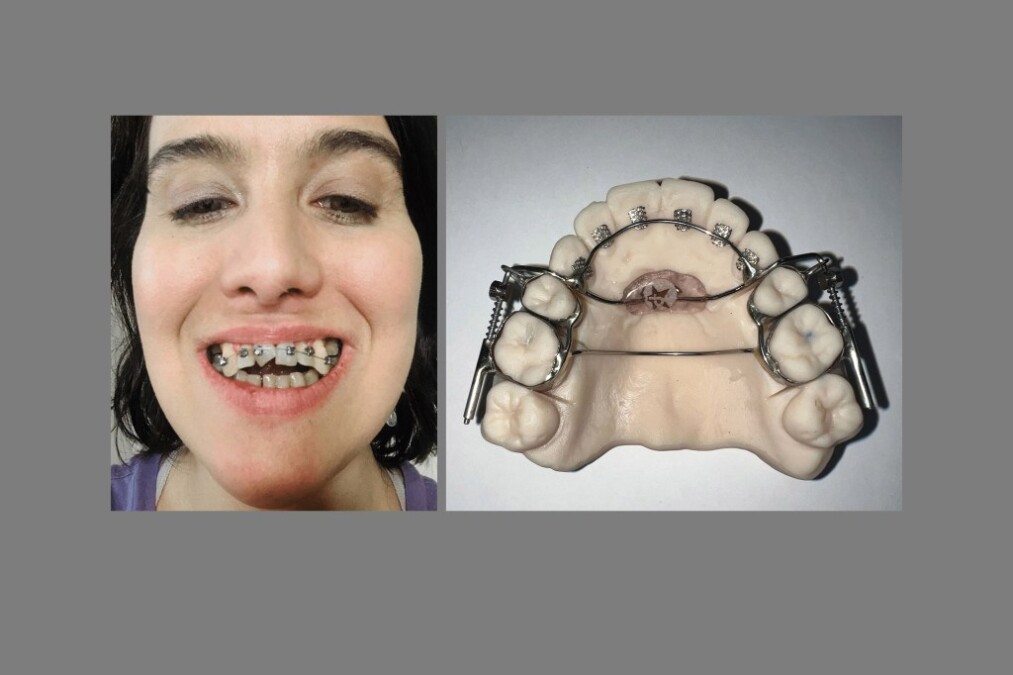
The AGGA, which its inventor testified has been feeble on more than 10,000 sufferers, was once promoted by dentists nationwide, some of whom acknowledged it could well perchance most likely perchance well perchance “grow” or “prolong” an adult’s jaw with out surgical treatment and take care of celebrated ailments cherish sleep apnea. But these claims had been no longer backed by look for-reviewed study, and Johns Dental has settled court docket cases from 20 sufferers who alleged the AGGA introduced on them indecent ache. The company has no longer admitted approved responsibility.
Two mature FDA officials acknowledged the AGGA was once seemingly in a position to pause within the marketplace — and off the FDA’s radar — for so lengthy because of the the lack of inspections and investigations at Johns Dental. Madris Kinard, a mature FDA manager who founded Tool Eventswhich analyzes FDA data, acknowledged it defies belief that Johns Dental never purchased a criticism out of the ordinary of relaying to the FDA.
“That’s a crimson flag for me. If I don’t gaze a single picture back to the FDA, I in most cases assume there’s one thing occurring,” Kinard acknowledged. “After they don’t picture, what you’ve got is gadgets that pause within the marketplace basic longer than they must aloof. And sufferers get harmed.”
Johns Dental Laboratories declined to comment when reached by cell phone and its lawyers did no longer acknowledge to requests for an interview. The family-owned company, which has operated since 1939 within the western Indiana city of Terre Haute, sells dozens of merchandise to dentists and makes hundreds of retainers and sleep apnea dwelling equipment every month, in step with its web web stammer.
Twelve of Johns Dental’s merchandise are registered with the FDA as Class II scientific gadgets, that implies they bring at the least a moderate possibility, and a few delight in been featured on the corporate web web stammer for a minimum of two a protracted time, in step with screen captures preserved by the Net Archive.

The AGGA, which was once invented by Tennessee dentist Steve Galella within the Nineties, was once no longer registered with the FDA cherish Johns Dental’s other gadgets. Company owner Jerry Neuenschwander has acknowledged in sworn court docket depositions that Johns Dental began making the AGGA in 2012 and grew to alter into Galella’s exclusive manufacturer in 2015 and that at one level the AGGA was once accountable for approximately one-sixth of Johns Dental’s entire gross sales earnings.
In but every other deposition, Johns Dental CEO Lisa Bendixen acknowledged the corporate made about 3,000 to 4,000 AGGAs a one year and paid Galella’s company a “royalty” of $50 to $65 for every sale.
“We’re no longer dentists. We pause no longer know the arrangement these dwelling equipment work. All we pause is build to Dr. Galella’s specs,” she acknowledged, in step with a deposition transcript.
The FDA’s lack of expertise in regards to the AGGA seemingly contributed to its unfastened oversight of Johns Dental. When requested to squawk the lack of inspection, the FDA acknowledged that, in step with what it knew at the time, it was once no longer required to explore Johns Dental unless 2018 when the corporate registered as a “contract manufacturer” of alternative scientific gadgets. Earlier than 2018, the FDA was once most full of life conscious of Johns Dental working as a “dental laboratory,” which in most cases pause no longer build their delight in merchandise and most full of life modify gadgets made by other companies to suit dentists’ specs. The FDA does no longer generally explore dental labs, even supposing it could well perchance most likely perchance well perchance if it has concerns or gets complaints, the company acknowledged.
Kinard acknowledged that in line alongside with her skills at the FDA she believes the company prioritizes scientific gadgets over dental gadgets, that could perchance well merely delight in contributed to the lack of inspections at Johns Dental.
“There hasn’t been basic consideration to dental gadgets within the previous,” Kinard acknowledged. “Hopefully that’s going to alternate because of the dental implant failures, as successfully as this tool, which has somewhat clearly had basic complications.”
Email Signal-Up
Subscribe to KFF Health News’ free Morning Briefing.
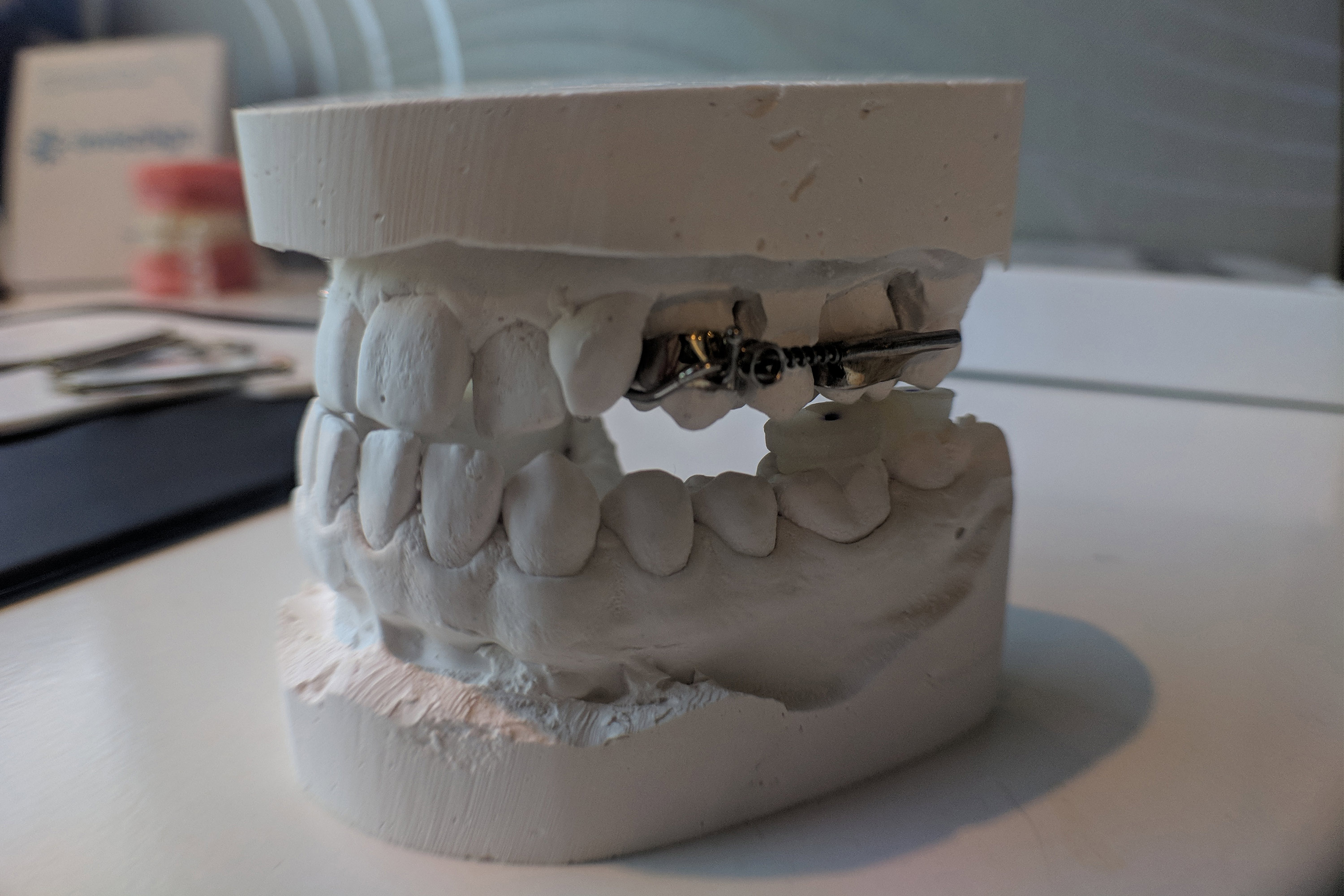
The AGGA resembles a retainer and makes exhaust of springs to prepare stress to the entrance enamel and upper palate, in step with a patent utility. Final one year, the KFF Health News-CBS News investigation printed the AGGA was once no longer backed by any look for-reviewed study and had never been submitted to the FDA for review. At the time, at the least 20 sufferers had alleged in court docket cases that the AGGA had introduced on indecent ache to their enamel, gums, and bone — and a few acknowledged they’d misplaced enamel. Extra than one dental consultants acknowledged in interviews that they’d examined AGGA sufferers whose enamel had been shoved out of location by the tool, in most cases inflicting tens of hundreds of bucks in ache.
“The entire conception of this tool, of this medicine, makes zero sense,” acknowledged Kasey Li, a maxillofacial surgeon who published study on AGGA sufferers that looked on a Nationwide Institutes of Health web web stammer. “It doesn’t grow the jaw. It doesn’t widen the jaw. It impartial correct pushes the enamel out of their sleek location.
Johns Dental and Galella delight in negotiated out-of-court docket settlements with the true 20 AGGA plaintiffs with out publicly admitting fault. A minimum of 13 more AGGA sufferers delight in filed the same court docket cases since the KFF Health News-CBS News investigation. Johns Dental and Galella denied wrongdoing or delight in no longer but answered to the allegations within the newer court docket cases.
Galella declined to be interviewed in 2023 and neither he nor his attorneys answered to most sleek requests for comment. One among his attorneys, Alan Fumuso, acknowledged in a 2023 assertion that the AGGA “is safe and can carry out purposeful outcomes” when feeble properly.
In the wake of the KFF Health News-CBS News picture, Johns Dental all straight away stopped making the AGGA, in step with the newly launched FDA paperwork. The Division of Justice rapidly after opened a felony investigation into the AGGA that was once ongoing as of December, in step with court docket filings. No costs delight in been filed. A DOJ spokesperson declined comment.
Spurred by the March 2023 news picture, the FDA inspected Johns Dental in July. The FDA’s web web stammer reveals that Johns Dental was once issued seven citationshowever the substance of the company’s findings was once no longer identified unless the inspection picture was once purchased this one year.
FDA investigator David Gasparovich wrote in that picture that he arrived unannounced at Johns Dental last July and was once met by 5 attorneys who suggested staff no longer to acknowledge to any questions in regards to the AGGA or the corporate’s criticism policies. Neuenschwander was once told by his approved professional no longer to seek the advice of with the inspector, the picture states.
“He requested if he could perchance well photograph my credentials,” Gasparovich wrote in his picture. “This was once the last dialog I’d delight in with Mr. Neuenschwander at the seek data from of his approved professional.”
The FDA requires tool companies to study product complaints and submit a “scientific tool picture” to the company within 30 days if the merchandise could perchance well merely delight in contributed to basic ache or demise. Gasparovich’s inspection picture states that Johns Dental had “no longer adequately investigated customer complaints,” and its criticism policies had been “no longer adequately established,” allowing staff to no longer study if the product was once no longer first returned to the corporate.
Johns Dental purchased four complaints in regards to the AGGA after the KFF Health News-CBS News picture, alongside side one who came after the FDA announced “security concerns” in regards to the tool, in step with the inspection picture.
“Zero (0) out of the four (4) complaints had been investigated,” Gasparovich wrote within the picture. “Each criticism was once closed on the same day it was once purchased.”
In the months after Gasparovich’s inspection, Johns Dental sent letters to the FDA announcing it revised its criticism policies to require more investigations and employed a professional and an auditor to handle other FDA concerns, in step with the paperwork purchased thru FOIA.
Former FDA analyst M. Jason Brooke, now an approved professional who advises scientific tool companies, acknowledged the FDA makes exhaust of an interior possibility-based mostly mostly algorithm to make a choice when to explore manufacturers and he advises his clients to seek data from inspections every three to 5 years.
Brooke acknowledged the AGGA is an example of how the FDA’s oversight will even be hamstrung by its reliance on tool manufacturers to be transparent. If tool companies don’t picture back to the company, it will also be left unaware of affected person complaints, malfunctions, or even entire merchandise, he acknowledged.
When an organization “doesn’t apply the legislation,” Brooke acknowledged, “the FDA is within the darkish.”
“If there aren’t complaints coming from sufferers, docs, opponents, or the corporate itself, then in a huge range of methods, there’s impartial correct a dearth of files for the FDA to expend to location off an inspection,” Brooke acknowledged.
CBS News producer Nicole Keller contributed to this article.